FDA Purple Book: What It Is and Why It Matters for Generic Drugs
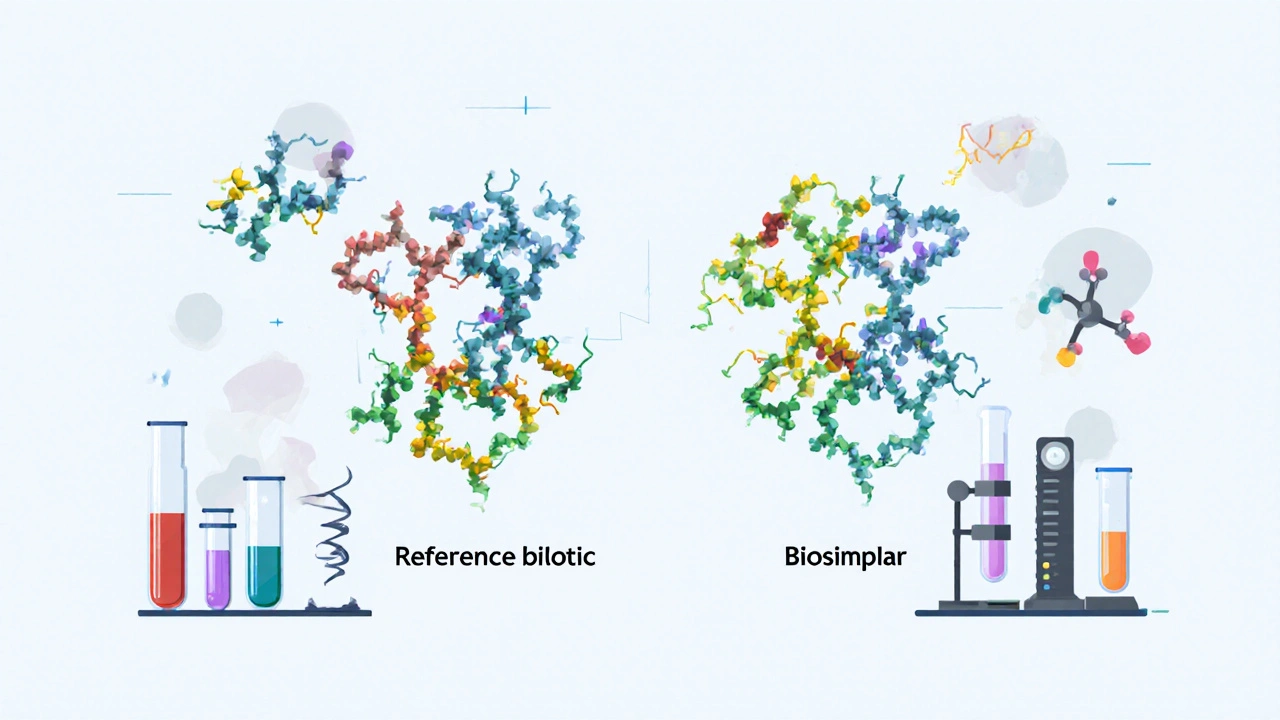
When you’re looking for a cheaper version of a biologic drug—like Humira or Enbrel—you’re not just shopping around. You’re relying on a government list called the FDA Purple Book, a public database maintained by the U.S. Food and Drug Administration that identifies approved biological products and their interchangeable biosimilars. Also known as Biologics and Biosimilars Approved Product List, it’s the official guide that tells pharmacists and doctors which generic versions of complex biologic drugs are legally approved and can be substituted safely. Unlike the Orange Book, which covers small-molecule generics like ibuprofen or metformin, the Purple Book deals with biologics—drugs made from living cells, not chemicals. These include treatments for cancer, arthritis, diabetes, and autoimmune diseases, and they’re often priced at $10,000 a year or more. The Purple Book is what makes affordable alternatives possible.
Biologics are harder to copy than regular pills. That’s why the FDA created a special category called biosimilars, drugs that are highly similar to an already-approved biologic, with no clinically meaningful differences in safety or effectiveness. The Purple Book lists each original biologic alongside every approved biosimilar, so you can see which ones are interchangeable—meaning a pharmacist can swap them without asking your doctor. For example, if your prescription says "Humira," the Purple Book tells you if a biosimilar like "Adalimumab-atto" is interchangeable. That’s huge for people paying out-of-pocket or fighting insurance denials. It’s also why you’ll see posts here about step therapy, drug costs, and switching medications—because the Purple Book is the hidden rulebook behind those decisions.
The FDA doesn’t just publish this list—it updates it every month. New biosimilars enter the market constantly, and some older ones get pulled or reclassified. If you’re managing a chronic condition like rheumatoid arthritis or Crohn’s disease, checking the Purple Book helps you stay ahead of pricing changes and treatment options. It’s not something most patients know about, but it’s why your co-pay might drop from $500 to $50 overnight. The posts below cover real-world impacts: how insurance uses this data to force generic switches, how hospitals track biosimilar use, and how patients navigate confusion when their pharmacist hands them a new bottle with a different name. This isn’t just paperwork—it’s your access to affordable care.
FDA Listing for Biosimilars: How They Are Evaluated and Approved
The FDA doesn't rate biosimilars like generics-they undergo a rigorous scientific review to prove they're highly similar to the original biologic with no clinically meaningful differences. Learn how approval works, why they're not interchangeable by default, and what's changing in 2025.
Read More