Understanding Bladder Cancer and Its Treatment Options
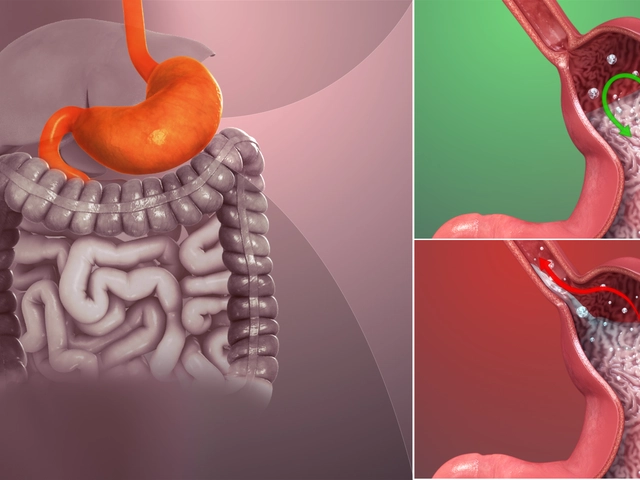
Before diving into the role of capecitabine in treating bladder cancer, it is essential to understand the basics of this disease. Bladder cancer is the growth of abnormal cells in the bladder lining, which can spread to other parts of the body if not treated in time. The most common type of bladder cancer is transitional cell carcinoma, which affects the cells lining the bladder. Treatment options for bladder cancer include surgery, radiation therapy, chemotherapy, and immunotherapy.
In this article, we will explore the role of capecitabine, a chemotherapy drug, in treating bladder cancer. We will discuss how this medication works, its potential benefits, and the possible side effects that patients may experience during treatment.
Capecitabine: An Overview of Its Mechanism and Use
Capecitabine is an oral chemotherapy drug that belongs to a group of medications called antimetabolites. It works by interfering with the DNA synthesis of cancer cells, which ultimately leads to cell death. This medication is typically used to treat various types of cancer, including colorectal, breast, and stomach cancer. However, recent research has shown that capecitabine may also be effective in treating bladder cancer.
Capecitabine is usually taken as a pill twice daily, with doses depending on the patient's body surface area and the specific type of cancer being treated. It is often given in combination with other chemotherapy drugs or radiation therapy to enhance its effectiveness.
Combining Capecitabine with Radiation Therapy
One of the ways capecitabine is utilized in bladder cancer treatment is by combining it with radiation therapy. This combination is known as chemoradiation, and it has been shown to improve treatment outcomes in some patients. Radiation therapy works by using high-energy rays to target and destroy cancer cells, while capecitabine helps to enhance the effectiveness of radiation by making cancer cells more sensitive to radiation damage.
Studies have indicated that chemoradiation with capecitabine can be an effective treatment option for patients with muscle-invasive bladder cancer, particularly for those who are not suitable candidates for surgery or other aggressive treatments.
Capecitabine as a Neoadjuvant Therapy
Neoadjuvant therapy refers to the use of medications or other treatments before the primary treatment, such as surgery or radiation therapy. Capecitabine can be used as a neoadjuvant therapy for bladder cancer patients to help shrink tumors and make them easier to remove surgically. This approach can potentially improve the chances of successfully removing the entire tumor and reducing the risk of cancer recurrence.
Research on the effectiveness of capecitabine as a neoadjuvant therapy for bladder cancer is still ongoing. However, initial studies have shown promising results, indicating that this treatment approach may be beneficial for some patients.
Capecitabine as an Adjuvant Therapy
Adjuvant therapy is a treatment given after the primary treatment, such as surgery or radiation therapy, to reduce the risk of cancer recurrence. Capecitabine can be used as an adjuvant therapy for bladder cancer patients who have undergone surgery or radiation therapy. The goal of this treatment is to eliminate any remaining cancer cells and lower the chances of the cancer coming back.
While the use of capecitabine as an adjuvant therapy for bladder cancer is still being studied, some research has suggested that it may be a beneficial treatment option for certain patients, particularly those with a high risk of recurrence.
The Potential Benefits of Capecitabine in Bladder Cancer Treatment
There are several potential benefits of using capecitabine in bladder cancer treatment, including:
- Improved treatment outcomes when used in combination with radiation therapy or other chemotherapy drugs
- Ability to shrink tumors before surgery, making them easier to remove
- Reducing the risk of cancer recurrence when used as an adjuvant therapy
- Oral administration, which is more convenient and comfortable for patients compared to intravenous chemotherapy
While more research is needed to fully understand the role of capecitabine in bladder cancer treatment, these potential benefits make it a promising option for some patients.
Possible Side Effects of Capecitabine
As with any medication, capecitabine can cause side effects in some patients. The most common side effects of capecitabine include:
- Diarrhea
- Nausea and vomiting
- Hand-foot syndrome (redness, swelling, and pain in the hands and feet)
- Fatigue
- Loss of appetite
- Mouth sores
While these side effects can be uncomfortable, they are generally manageable with proper care and support from your healthcare team. It is essential to discuss any concerns or side effects with your doctor, who can help determine the best course of action for managing these symptoms.
Conclusion: The Future of Capecitabine in Bladder Cancer Treatment
Although more research is needed to fully understand the role of capecitabine in bladder cancer treatment, the existing evidence suggests that this medication may be a valuable addition to the treatment options available for some patients. Its potential benefits, such as improved treatment outcomes, tumor shrinkage, and reduced risk of recurrence, make it a promising option for those with bladder cancer.
As research continues, we will likely gain a better understanding of the most effective ways to use capecitabine in bladder cancer treatment, as well as the specific patient populations that may benefit the most from this medication. In the meantime, it is essential to discuss any treatment options, including capecitabine, with your healthcare team to determine the best course of action for your unique situation.




SandraAnn Clark
April 27, 2023 AT 17:24Capecitabine sounds promising, but who really cares?
Patrick Vande Ven
April 28, 2023 AT 21:11The mechanism of capecitabine, as an antimetabolite, is well‑documented in the oncology literature. It undergoes enzymatic conversion to 5‑fluorouracil preferentially within tumor cells, thereby limiting systemic exposure. When combined with radiation, the drug acts as a radiosensitiser, enhancing DNA damage inflicted by ionising photons. Several phase II studies have reported improved local control rates in muscle‑invasive bladder cancer when capecitabine‑based chemoradiation is employed. However, the heterogeneity of patient populations in these trials warrants cautious interpretation of the results.
Akshay Pure
April 30, 2023 AT 06:31The ontological implications of capecitabine's integration into bladder carcinoma protocols merit rigorous exegesis. Its pharmacokinetic profile, characterized by enzymatic activation within neoplastic tissues, ostensibly confers a therapeutic index superior to traditional intravenous analogues. Moreover, the synergistic potentiation observed when co‑administered with ionizing radiation invokes a paradigm wherein cytotoxicity is spatially concentrated. Clinical trials, albeit nascent, have delineated response rates that eclipse historical baselines for muscle‑invasive phenotypes. Nonetheless, the heterogeneity of tumor microenvironments predicates a stratified approach to patient selection. Molecular biomarkers, such as thymidine phosphorylase expression, may forecast susceptibility to capecitabine‑mediated apoptosis. Equally salient is the mitigation of systemic toxicity, a corollary of oral administration obviating the need for central venous access. Yet, the specter of hand‑foot syndrome looms, demanding prophylactic dermatologic interventions. Hematologic derangements, though infrequent, necessitate vigilant surveillance. The economic calculus, factoring drug acquisition costs against inpatient expenditures, suggests a favorable cost‑effectiveness ratio in selected cohorts. Ethical considerations arise when extrapolating data from colorectal oncology to urological malignancies, underscoring the necessity for disease‑specific trials. Patient-reported outcomes have illuminated an appreciation for the convenience of ambulatory dosing, albeit tempered by concerns regarding adherence. The confluence of neoadjuvant and adjuvant applications positions capecitabine as a versatile agent within multimodal regimens. Future investigations should prioritize randomized controlled designs to substantiate its incremental benefit. In sum, while the preliminary evidence is compelling, a circumspect integration into standard care pathways remains prudent.
Matt Stone
May 1, 2023 AT 04:44Looks like the data is solid enough to try this drug with radiation its a win
Joy Luca
May 2, 2023 AT 00:11Indeed the pharmacodynamics align with synergistic radiosensitisation – the double‑hit hypothesis is well supported by preclinical models. Incorporating capecitabine into the neoadjuvant schema can compress tumour volume, facilitating organ‑preserving surgery. Just ensure dose‑modification algorithms are in place to mitigate hand‑foot syndrome and mucositis.
Jessica Martins
May 3, 2023 AT 01:11Common adverse events such as diarrhea, nausea, and hand‑foot syndrome should be monitored closely, with dose adjustments as needed. Early intervention with topical agents can reduce the severity of dermatologic toxicity.
Jeremy Olson
May 3, 2023 AT 17:51We appreciate the emphasis on proactive side‑effect management; patients often feel reassured when clinicians outline clear mitigation strategies. Maintaining quality of life during treatment is just as important as oncologic control.
Ada Lusardi
May 5, 2023 AT 03:11Wow this sounds hopeful 😃💪❤️
Scott Davis
May 6, 2023 AT 01:24Interesting read, will keep an eye on future trials.