For patients taking warfarin, a tiny change in blood concentration can mean the difference between life and death. Yet, when pharmacists substitute generic versions of this critical drug, many doctors worry about the consequences. This isn't just theoretical—real-world data shows how prescriber attitudes toward NTI drugs are medications where small changes in dose or blood concentration can lead to serious therapeutic failures or adverse reactions, with the FDA defining them as drugs where the ratio between the minimum toxic concentration and the minimum effective concentration is less than or equal to two. shape treatment decisions daily.
What Are Narrow Therapeutic Index (NTI) Drugs?
NTI drugs are medications where the difference between a safe dose and a toxic dose is very small. The FDA defines them as drugs where the ratio between the minimum toxic concentration and minimum effective concentration is two or less. This narrow margin means even tiny changes in blood levels can cause serious problems. For example, warfarin (a blood thinner) requires precise dosing; too little won't prevent clots, while too much can cause dangerous bleeding. Similarly, levothyroxine for thyroid issues needs exact levels to avoid symptoms like fatigue or heart palpitations. These drugs aren't just any medications—they're critical treatments where precision matters most.
Regulatory Frameworks and Bioequivalence Standards
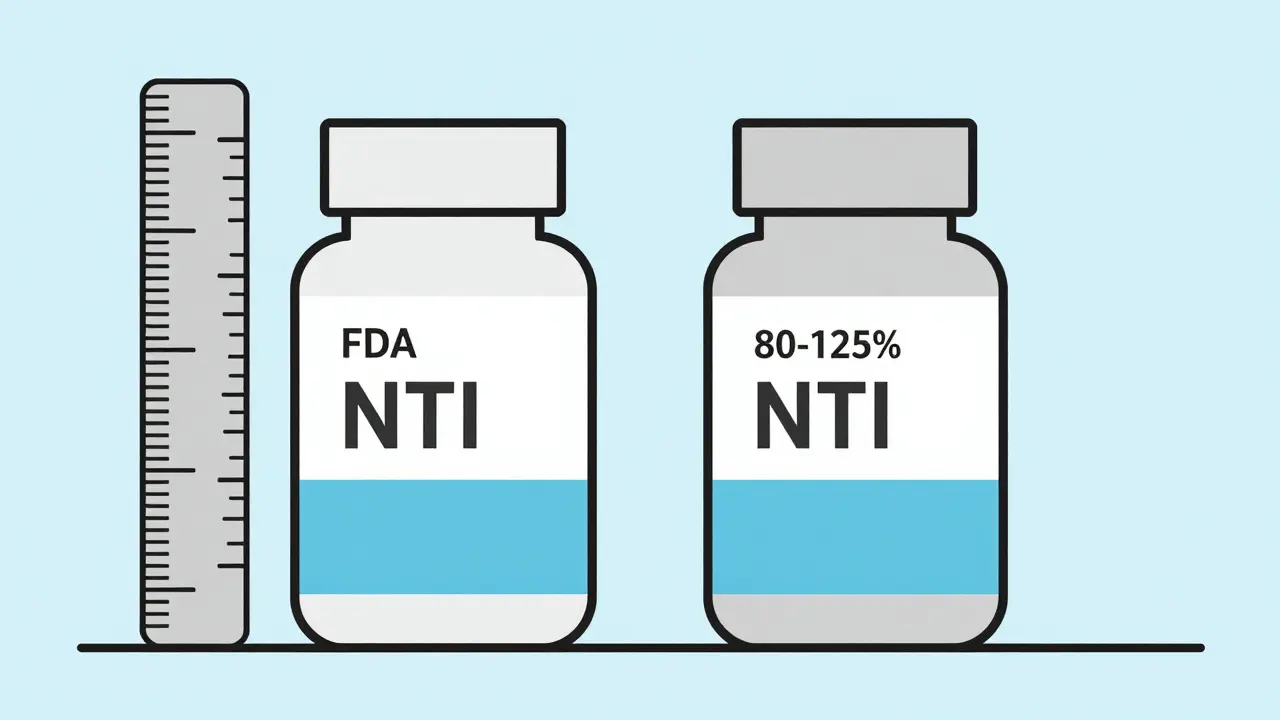
The FDA's approach to NTI drugs has evolved. The Hatch-Waxman Act of 1984 created the modern generic drug approval system but raised concerns about NTI drugs. In 2019, the FDA updated its guidance, requiring a tighter bioequivalence range (90-111%) for NTI generics instead of the standard 80-125%. This means generic versions must be much closer to the brand-name drug in how they're absorbed. However, even with these stricter standards, some prescribers remain skeptical. For example, transplant specialists argue that bioequivalence testing in healthy volunteers doesn't reflect real-world patient responses, as shown in a 1997 survey of 59 transplant pharmacists where 92% believed testing should happen in actual patients.
Prescriber Perspectives Across Specialties
Not all doctors share the same views on NTI drug substitution. The American Medical Association (AMA) has stated since 2007 that a more stringent substitution process is unnecessary, but real-world data tells a different story. A 2018 survey in Clinical Pharmacology & Therapeutics found that 68% of physicians worried about substituting generic warfarin, and 42% specifically feared INR fluctuations. Transplant specialists, who deal with drugs like tacrolimus, are even more cautious. A 2023 survey by the American College of Physicians found 57% of internists would prescribe brand-name NTI drugs for high-risk patients, citing stability concerns as the top reason. These differences highlight how specialty-specific risks shape clinical decisions.
State Laws Shape Substitution Practices
State regulations play a major role in NTI drug substitution. As of 2023, 28 U.S. states have specific rules for NTI drugs. Texas and Florida maintain official NTI drug lists that restrict automatic substitution, requiring prescriber notification or consent. A 2022 analysis in the Journal of Managed Care & Specialty Pharmacy showed states with "affirmative patient consent" laws (17 states as of 2022) had 23% lower generic NTI substitution rates compared to states without such requirements. This means where laws are stricter, doctors and pharmacists work together more closely to ensure safe transitions.
Communication Between Doctors and Pharmacists
Clear communication is key when NTI drugs are involved. A 2021 study in the Journal of the American Pharmacists Association found 63% of physicians prefer electronic notifications about substitutions over phone calls. Primary care doctors receive about 2.7 NTI substitution alerts monthly, while psychiatrists managing lithium prescriptions get 5.4. The AMA reports 41% of physicians experienced patient confusion after substitutions in 2022, leading to 29% more office visits for monitoring. Each incident costs an estimated $127 according to MGMA data. When doctors and pharmacists communicate effectively, these issues drop significantly.

Market Trends and Economic Impact
Market data reveals prescriber preferences. Medicare Part D data from 2022 shows brand-name NTI drugs hold 23% market share despite generic availability, compared to just 8% for non-NTI drugs. The top five NTI drugs by brand persistence are tacrolimus (32% brand share), warfarin (28%), levothyroxine (25%), phenytoin (21%), and lithium (19%). While the Congressional Budget Office estimates restricting substitution could increase Medicare spending by $1.2 billion annually, the Association for Accessible Medicines projects $127 billion in savings over 10 years with increased generic use. This tension between safety concerns and cost savings continues to shape policy debates.
Recent Developments and Future Outlook
Changes are underway. The FDA added 12 new drugs to the NTI list in March 2023 while removing three based on new evidence. The American Society of Clinical Oncology (ASCO) now supports generic substitution for oral oncology NTI drugs with therapeutic drug monitoring. The PRESCRIPT-NTI trial, enrolling 1,200 patients across 42 sites, is tracking clinical outcomes after substitution, with results expected in 2024. CMS proposed rules require prescriber notification for all NTI substitutions in Medicare Part D. Industry analysts predict NTI generic penetration will reach 78% by 2028 as real-world evidence builds trust in generic alternatives.
Key Takeaways
- Narrow Therapeutic Index (NTI) drugs have a very small margin between effective and toxic doses, making precise dosing critical.
- Despite FDA assurances, many physicians remain cautious about substituting generic versions of NTI drugs like warfarin and levothyroxine.
- 28 U.S. states have specific rules for NTI drug substitution, with some requiring prescriber consent before switching generics.
- Electronic notifications are preferred by 63% of physicians for substitution alerts, reducing confusion and monitoring needs.
- Brand-name NTI drugs maintain higher market share than non-NTI drugs, with tacrolimus at 32% brand usage in Medicare Part D.
What are Narrow Therapeutic Index (NTI) drugs?
NTI drugs are medications where the difference between a safe dose and a toxic dose is very small. The FDA defines them as drugs where the ratio between the minimum toxic concentration and minimum effective concentration is two or less. Examples include warfarin (blood thinner), levothyroxine (thyroid medication), phenytoin (seizure control), and lithium (bipolar treatment). Even minor changes in blood levels can lead to treatment failure or serious side effects.
Why do some doctors prefer brand-name NTI drugs over generics?
Many doctors worry about small variations in generic versions affecting patient outcomes. For example, a 2018 survey found 68% of physicians were concerned about substituting generic warfarin due to potential INR fluctuations. Transplant specialists, who rely on drugs like tacrolimus, often cite stability concerns. Brand-name drugs have consistent manufacturing, while generics must meet bioequivalence standards, but some clinicians prefer the known performance of the original product for critical treatments.
How do state laws affect NTI drug substitution?
Twenty-eight U.S. states have specific NTI drug substitution rules. Some, like Texas and Florida, maintain official lists of NTI drugs where automatic substitution is restricted, requiring prescriber consent. States with "affirmative patient consent" laws (17 as of 2022) saw 23% lower generic substitution rates. These laws ensure doctors are involved in substitution decisions, reducing risks for patients on critical medications.
What role do pharmacists play in NTI drug substitution?
Pharmacists are on the front lines of substitution decisions. A 2021 survey by ASHP found 78% of hospital pharmacists always notify prescribers before substituting NTI generics. They also monitor for potential issues and communicate with doctors about any changes. However, the American Society of Health-System Pharmacists emphasizes that substitution decisions should involve both pharmacists and prescribers working together based on clinical evidence, not just automatic switching.
Are there risks with substituting NTI drugs?
Yes, but the risks vary. The Institute for Safe Medication Practices documented 1,247 NTI-related medication errors between 2015-2020, with 37% involving substitution issues. However, only 8% of these errors caused patient harm. Common risks include fluctuations in blood levels leading to treatment failure (e.g., seizures from phenytoin changes) or toxicity (e.g., bleeding from warfarin). Proper monitoring and communication between healthcare providers can minimize these risks significantly.
What's the FDA's current stance on NTI drug generics?
The FDA maintains that generic NTI drugs are safe and effective. In 2020, their Center for Drug Evaluation and Research reported 98% of generic drugs, including NTI types, perform within 3-4% of brand-name versions based on post-market data. FDA Director Janet Woodcock stated in 2019 that the agency has strengthened monitoring of generic NTI drugs and improved communication with providers. However, the FDA also updated its guidance in 2023, adding 12 new drugs to the NTI list while removing three based on new evidence, showing ongoing evaluation of these medications.




Bella Cullen
February 4, 2026 AT 23:16I've been a nurse for 15 years. Warfarin generics? No way. My patients' INR goes wild.
Docs need to push back on this. FDA says it's fine but reality's different. Just saying.
Lana Younis
February 5, 2026 AT 23:41Hey folks, NTI drugs are tricky. The FDA's 90-111% range is okay but not perfect. I've seen cases where generics caused issues. But we need to talk about it, not just assume. Docs and pharmacists should collaborate. Maybe more real-world studies?
Also, warfarin's tricky because of diet interactions. It's not just the drug. Bioequivalance studies don't account for all variables. For example, some patients metabolize drugs differently. We need better data. Also, the manufacturing process for generics varies. A lot of people don't realize that. It's not just about the active ingredient. Excipients matter too. And let's not forget the cost savings. But safety first. I think we need more collaboration between all healthcare providers. Maybe a national registry for NTI drug outcomes? That could help track issues. Also, electronic alerts should be mandatory. Right now, they're not always used. This is a big problem.
Samantha Beye
February 7, 2026 AT 07:50Data shows generics are safe for most patients; better monitoring is the solution.
one hamzah
February 7, 2026 AT 15:43Wow! This is such an important topic. 🌟 NTI drugs need careful handling but generics are usually safe. Let's trust the science and keep improving communication! 💯 Also, the FDA's updates show they're on top of things. More studies are needed, but the current system works well. 💪
Andre Shaw
February 9, 2026 AT 09:52The FDA's current guidelines on NTI drugs are completely inadequate. They claim the 90-111% bioequivalence range is sufficient, but real-world data tells a different story. In my years as a clinical pharmacist, I've seen multiple cases where patients on generic tacrolimus had severe rejection episodes. The problem is that bioequivalence studies are done on healthy volunteers, not actual transplant patients who have different metabolic rates. Also, the manufacturing process for generics can vary between batches, leading to inconsistent drug absorption. This isn't just theoretical-there's documented evidence of increased adverse events with certain generic versions. The FDA needs to require more rigorous testing, including in-patient studies and stricter manufacturing controls. Until then, doctors should avoid substituting generics for NTI drugs whenever possible. The cost savings aren't worth risking patient lives. I've spoken to colleagues across the country, and we're all frustrated with the current system. The Congressional Budget Office's estimate of $1.2 billion in savings is irrelevant if patients are getting harmed. We need to prioritize safety over cost. This isn't about being anti-generic; it's about ensuring that the generics that are approved are truly safe for critical medications. For example, a 2022 study in the Journal of Transplantation showed a 15% higher rejection rate with certain generics. It's time for the FDA to take this seriously.
Jennifer Aronson
February 9, 2026 AT 15:15Effective communication between pharmacists and physicians is indeed paramount. However, the current systems for substitution alerts are inconsistent. A standardized electronic notification system could reduce errors significantly. Also, state laws vary too much-national guidelines would help.
Gregory Rodriguez
February 11, 2026 AT 00:06Oh sure, let's all panic about generics. The FDA's been monitoring these drugs for decades and they're perfectly safe. But hey, if you want to pay triple the price for brand name, go right ahead. 🤦♂️
Johanna Pan
February 12, 2026 AT 14:48I agree with the need for better communication. But sometimes the data is clear-some generics do cause issues. For example, a 2021 study in JAMA showed higer variablity in phenytoin levels with certain generics. We need to balance cost and safety. Maybe a tiered approach where high-risk patients get brand name?
Elliot Alejo
February 13, 2026 AT 19:05Collaboration between pharmacists and prescribers is key. We should share data on substitution outcomes to build trust. Let's focus on evidence-based practices instead of fear. It's the right move for patients.