When you pick up a generic pill, you expect it to work just like the brand-name version. But how do manufacturers prove it? In the past, they’d copy the formula, run a few tests at the end of production, and hope it passed. Today, that’s no longer enough. The industry has shifted to Quality by Design (QbD)-a science-driven, proactive way to build quality into generic drugs from day one. This isn’t just a trend. It’s now a regulatory requirement for nearly all new generic applications submitted to the FDA and EMA.
What Is Quality by Design (QbD) and Why It Matters
QbD isn’t about testing your way to quality. It’s about designing quality in. The International Council for Harmonisation (ICH) defines it as a systematic approach that starts with clear goals, uses scientific understanding, and manages risks to ensure the product consistently performs as intended. For generic drugs, that means proving bioequivalence not just through clinical trials, but through deep, measurable science.
Before QbD, manufacturers followed a recipe: mix for 15 minutes, compress at 12 kN, dry at 45°C. If the final product failed a dissolution test, they’d tweak the recipe and try again. No one knew why it failed-just that it did. QbD flips that. Instead of fixed settings, you define a range of acceptable conditions-called a design space-where the product will always meet quality standards. That’s why the FDA now requires QbD elements in every Abbreviated New Drug Application (ANDA) submitted after October 1, 2017.
The payoff? Approval rates jumped 23% and review times dropped by nearly five months per application, according to the FDA’s 2022 report. Companies also save $1.2-2.8 million annually per product by avoiding costly regulatory filings for minor process changes. That’s not just efficiency-it’s survival in a low-margin industry.
The Five Pillars of QbD in Generic Development
QbD isn’t a buzzword. It’s a structured framework with five non-negotiable components:
- Quality Target Product Profile (QTPP): This is your destination. It lists every critical characteristic the generic must match: identity, strength, dissolution profile, impurity levels. The FDA requires at least 95% similarity to the Reference Listed Drug (RLD) in dissolution testing.
- Critical Quality Attributes (CQAs): These are the measurable traits that impact safety and effectiveness. For most generics, you’ll identify 5-12 CQAs. Common ones include dissolution rate (f2 similarity factor >50), content uniformity (RSD ≤6.0%), and impurity limits under ICH Q3B guidelines.
- Critical Process Parameters (CPPs): These are the levers you control during manufacturing. Through Design of Experiments (DoE), you test how changes in granulation moisture (1.5-3.0%), compression force (10-15 kN), or drying temperature (40-50°C) affect your CQAs. You don’t guess-you prove.
- Design Space: This is the multidimensional zone where all CPPs combine to guarantee quality. The FDA accepts design spaces built on 100+ simulated batches, with 95% confidence that CQAs stay within limits. Once approved, you can move within this space without seeking prior regulatory approval.
- Control Strategy: This includes how you monitor and manage quality during production. Most QbD-ready manufacturers now use Process Analytical Technology (PAT)-like near-infrared spectroscopy-to test tablets mid-process. This cuts end-product testing by 35-60%, according to the Parenteral Drug Association.
Each of these pillars is linked. Change the CPPs? You must show it doesn’t break the CQAs. Adjust the design space? You need data to back it. This isn’t paperwork-it’s science.
QbD vs. Traditional Development: The Real Difference
Traditional development is reactive. You build it, test it, fix it. QbD is predictive. You build it right the first time because you understand how every variable affects the outcome.
A 2023 Tufts study of 127 generic products found QbD methods improved process robustness by 28-42% during scale-up. Why? Because you’re not locking in one setting-you’re understanding a range. If your mixer runs hotter than planned? In a traditional model, that’s a failure. In a QbD model, if it’s still inside your design space, it’s perfectly acceptable.
Regulatory outcomes reflect this. The FDA’s Office of Generic Drugs reports QbD-based ANDAs get 31% fewer Complete Response Letters (CRLs). Approval timelines average 9.2 months versus 13.9 months for non-QbD submissions. That’s a 34% faster path to market.
But it’s not all smooth. QbD adds 4-8 months to development timelines and increases upfront costs by 25-40%. For a simple immediate-release tablet, that’s often unnecessary. As Dr. James Polli of the University of Maryland warned, over-engineering QbD for low-complexity products can cost $450,000 in DoE studies that add no real value.
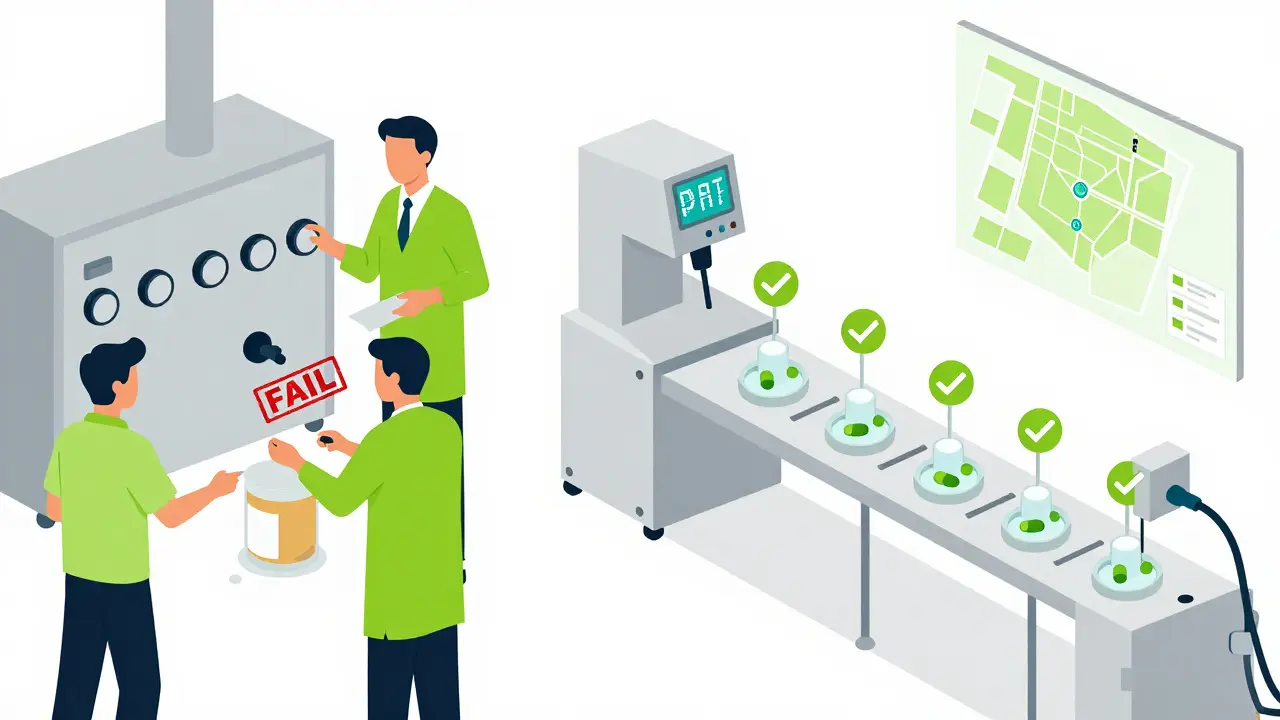
Where QbD Shines-and Where It Struggles
QbD isn’t a one-size-fits-all tool. It’s most powerful for complex generics: inhalers, transdermal patches, modified-release tablets, and injectables. These products have tricky bioequivalence challenges. Traditional methods often fail to predict real-world performance. QbD bridges that gap with in vitro-in vivo correlations (IVIVC), which link lab dissolution data to how the drug behaves in the body.
But here’s the catch: 63% of QbD failures in generic development come from poor mechanistic understanding, especially with complex formulations. The EMA found that 22% of applicants can’t establish reliable IVIVC for extended-release products. That’s not a failure of QbD-it’s a failure to invest in the science behind it.
For ultra-low-cost generics-think $0.01-per-pill products-QbD can be a financial burden. If your product’s lifetime revenue is under $50 million, spending $1 million on development isn’t sustainable. The Generic Pharmaceutical Association warns that QbD must be scaled proportionally. For simple tablets, a lean QbD approach with minimal DoE and strong RLD comparison may be enough.
Real-World Impact: What Companies Are Saying
Dr. Elena Rodriguez at Hikma Pharmaceuticals cut post-approval deviations for her generic esomeprazole from 14 to 2 per year after switching to QbD. That saved $850,000 annually in quality investigations.
At Mylan (now Viatris), Dr. Sarah Kim used QbD to make 11 manufacturing adjustments to their simvastatin process without seeking FDA approval. During pandemic supply chain chaos, that kept delivery rates at 99.8%.
But it’s not all wins. Dr. Mark Chen at Lupin Limited spent 120 person-hours training his team on QbD principles. The first two ANDA submissions stalled because the team wasn’t ready. The learning curve is steep.
According to the 2023 Generic Pharmaceutical Association survey, 78% of companies say regulatory interactions improved after QbD. Sixty-three percent report fewer questions during Type B meetings. But 52% still struggle to justify design space boundaries for multi-component products.
How to Implement QbD: A Practical Roadmap
There’s no shortcut, but there is a proven path:
- Start with the RLD. Use advanced analytical tools to fully characterize the reference drug. This can cut development time by 30%.
- Define your QTPP. Match dissolution, strength, and impurity profiles to the brand. Don’t assume-they’re your baseline.
- Identify CQAs. Focus on what matters: dissolution, uniformity, stability. Don’t over-test.
- Use DoE to map CPPs. Test variables like moisture, pressure, and temperature in a structured way. Tools like MODDE Pro help analyze multivariate data.
- Build and validate your design space. Run simulations. Use PAT tools for real-time monitoring. Aim for 95% confidence in CQA compliance.
- Implement a control strategy. Combine PAT, in-process checks, and end-product testing. Reduce reliance on final batch testing.
Expect to invest: $500,000+ in PAT equipment, $15,000/year per user for analysis software, and 80-120 hours of training per scientist in QbD and risk management (ICH Q9). The FDA’s QbD Pilot Program has approved 87 submissions with a 92% first-cycle approval rate-far above the 78% for traditional submissions.
The Future of QbD: Where It’s Headed
QbD is evolving fast. The FDA’s new ICH Q14 guideline (effective December 2023) requires more robust analytical method validation-but rewards it with 40% faster approval for QbD-aligned submissions. The agency’s Emerging Technology Program has approved 27 QbD-based continuous manufacturing applications with 100% success.
By 2027, McKinsey predicts 95% of new generic approvals will include QbD. The WHO now includes QbD criteria in its prequalification program, pushing global harmonization. Indian manufacturers, despite cost pressures, invested $227 million in QbD capabilities in 2022. The top 10 companies there are now on par with U.S. and EU peers.
Emerging areas? 3D-printed generics and complex biologics follow-ons. These will need even deeper QbD integration. The message is clear: if you’re not building quality in, you’re not building for the future.
Final Thoughts: Is QbD Worth It?
Yes-if you do it right. For complex generics, it’s non-negotiable. For simple tablets, it’s optional-but smart. The goal isn’t to do the most studies. It’s to do the right ones. Use QbD to reduce risk, speed approvals, and avoid costly recalls. Don’t treat it as a regulatory hurdle. Treat it as your competitive edge.
The days of guessing your way to bioequivalence are over. The science is here. The regulators expect it. The market rewards it. If you’re developing generics in 2025, you’re not just making pills-you’re building science.
What is the main goal of Quality by Design in generic drug development?
The main goal of Quality by Design (QbD) is to build quality into generic drugs from the start, using scientific understanding and risk management, rather than testing for quality only after manufacturing. This ensures consistent product performance, reduces regulatory surprises, and allows flexibility in manufacturing within approved design spaces.
How does QbD improve bioequivalence testing for generics?
QbD improves bioequivalence by linking in vitro data-like dissolution profiles-to real-world performance using in vitro-in vivo correlations (IVIVC). Instead of relying solely on clinical trials, manufacturers can prove equivalence through scientifically validated lab tests, which are more consistent, repeatable, and cost-effective.
What are Critical Quality Attributes (CQAs) in QbD?
Critical Quality Attributes (CQAs) are measurable physical, chemical, biological, or microbiological properties that must be within an appropriate limit to ensure the drug’s safety and effectiveness. For generics, common CQAs include dissolution rate (f2 >50), content uniformity (RSD ≤6.0%), and impurity levels per ICH Q3B guidelines.
Is QbD required by the FDA for all generic drugs?
Yes. Since October 1, 2017, the FDA requires QbD elements in all Abbreviated New Drug Applications (ANDAs). While not every step must be equally detailed, the core components-QTPP, CQAs, design space, and control strategy-must be clearly defined and scientifically justified.
How does QbD reduce manufacturing costs over time?
QbD reduces long-term costs by allowing manufacturers to make process changes within an approved design space without prior FDA approval. This avoids costly regulatory submissions, cuts down on post-approval deviations, and reduces the need for end-product testing. Companies report annual savings of $1.2-2.8 million per product.
Can small generic manufacturers afford to implement QbD?
Yes, but they must scale it appropriately. For simple immediate-release tablets, a lean QbD approach using existing RLD data and minimal DoE studies can be cost-effective. For low-revenue products under $50 million annually, the development cost should stay under 15% of projected lifetime revenue. Outsourcing or partnering with QbD consultants is also a viable option.
What tools are essential for QbD implementation?
Essential tools include Process Analytical Technology (PAT) like near-infrared spectroscopy for real-time monitoring, multivariate analysis software (e.g., MODDE Pro), and equipment for dissolution testing, particle size analysis, and impurity profiling. Training in ICH Q9 (risk management) and Design of Experiments (DoE) is also critical.
How does QbD compare to traditional generic development?
Traditional development uses fixed, recipe-based manufacturing with single-point parameters. Quality is verified only at the end. QbD uses scientific understanding to define operating ranges (design space), monitors quality during production, and allows flexibility without regulatory approval. This results in higher robustness, faster approvals, and fewer regulatory setbacks.
What are the biggest challenges in implementing QbD?
The biggest challenges are high upfront costs, extended development timelines (4-8 months longer), lack of mechanistic understanding-especially for complex products-and difficulty justifying design space boundaries. Training staff and securing investment in advanced equipment are also major hurdles.
Is QbD used globally, or just in the U.S. and Europe?
QbD is now a global standard. The FDA, EMA, and PMDA (Japan) require it for complex generics. The WHO includes QbD criteria in its prequalification program, and countries like India and Brazil are rapidly adopting it. Even manufacturers in emerging markets are investing heavily to meet international quality expectations.



Janette Martens
December 31, 2025 AT 02:40QbD? More like Quality by Bureaucracy. We’re paying $20 for a pill and now they want us to fund $1M in spectroscopy just so some PhD can tweak a drying temp? I’d rather take my chances than fund this over-engineered circus.
Paige Shipe
January 2, 2026 AT 01:30The notion that QbD reduces regulatory delays is statistically valid, but the underlying assumption-that all manufacturers possess the technical infrastructure to implement PAT or DoE-is fundamentally flawed. The FDA’s 92% first-cycle approval rate applies only to entities with validated analytical systems and trained personnel, which excludes the majority of global generic producers. This creates a de facto barrier to entry that favors large conglomerates and undermines market competition.
Duncan Careless
January 2, 2026 AT 16:22It’s funny how we treat QbD like it’s some revolutionary breakthrough, when really it’s just good science dressed up in regulatory jargon. The real win isn’t the 23% higher approval rate-it’s that companies finally stopped guessing. Still, I worry we’re applying this like a hammer to every nail. A simple 500mg aspirin doesn’t need a 100-batch DoE. Sometimes, less is more.
Greg Quinn
January 3, 2026 AT 00:20There’s a quiet beauty in QbD-it’s the first time in pharma that we’ve treated a drug like a system instead of a product. We don’t just want the pill to work; we want to understand why it works, how it fails, and what keeps it stable. That’s not compliance. That’s epistemology. The cost? Sure. But the alternative-blind trust in a tablet-is the real risk.
Marie-Pierre Gonzalez
January 3, 2026 AT 12:46As someone who’s seen generics go from $0.01 to $0.50 overnight due to supply chain chaos, I can say this: QbD saved our lives. We had a 3-day window to switch suppliers during the pandemic, and because our design space allowed for a 10% variance in compression force, we didn’t miss a single shipment. Thank you, science. 🙏
Manan Pandya
January 4, 2026 AT 19:26India’s investment of $227 million in QbD infrastructure is a strategic masterstroke. While Western firms focus on cost avoidance, Indian manufacturers are building capability. The real test will be whether they can sustain this without regulatory capture. For now, the data speaks: 8 of the top 10 global ANDA approvals in 2023 came from Indian firms using QbD. The future isn’t American or European-it’s global, and it’s data-driven.
Aliza Efraimov
January 5, 2026 AT 22:30I worked on a transdermal patch that failed 3 times because they used a ‘standard’ formulation from 2012. QbD didn’t just fix it-it saved the product line. We built an IVIVC model that predicted in vivo performance with 94% accuracy. That’s not luck. That’s precision. And yes, it cost $800K. But when your drug treats 2 million people, you don’t cut corners-you build bridges.
Nisha Marwaha
January 5, 2026 AT 23:43Let’s not conflate QbD with complexity. The core tenets-QTPP, CQAs, design space, control strategy-are not novel; they’re foundational. The innovation lies in their formalization and integration. What’s often missed is that QbD is not a methodology-it’s a paradigm shift from empirical to mechanistic. Without understanding the physicochemical drivers of dissolution, you’re not doing QbD-you’re doing placebo science.
Tamar Dunlop
January 7, 2026 AT 13:36As a Canadian pharmacist who’s seen patients ration insulin because generics vanish from shelves, I can tell you this: QbD isn’t about paperwork. It’s about reliability. When your life depends on a pill working the same way every day, you don’t want a recipe-you want a guarantee. That’s what QbD delivers. And yes, it’s expensive. But so is a hospital bed.